GMP Planning and Consulting
The implementation of complex pharmaceutical projects requires a holistic planning and an intensive consulting service on the highest level.
All our service aims at efficiency, functional reliability and acceptance by the authorities. Correspondence to the current GMP guidelines of EU, FDA, PIC und WHO is of prime importance for us.
GxP - Planning and Consulting
- Conceptual Design Studies,Feasibility Studies
- Master Plans, Complete Planning
- Project Controlling, Project Management
- Planning of GxP Laboratory Areas
- Planning of GMP-conform Production Areas
- Basic and Detail Engineering
- GMP-conform Texts for Tendering
- Site Supervision
- GMP Acceptance Procedures
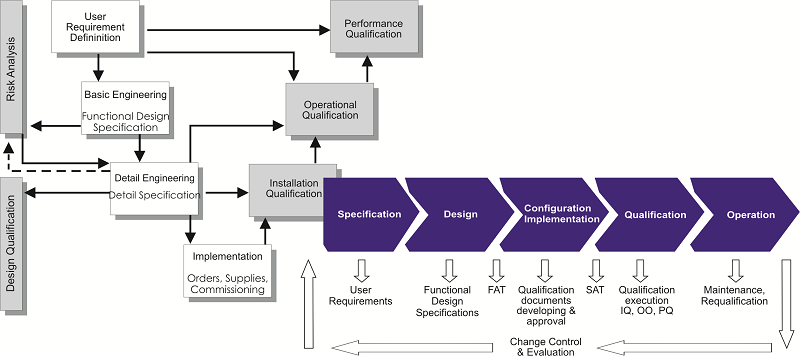
GxP - Validation and Qualification
- User Requirement Specifications (URS)
- Validation Master Plans (VMP)
- Qualification Master Plans (QMP)
- Site Master Files (SMF)
- Design Specifications (DQ)
- Risk Analysis
- Qualification Protocols, Reports and Summaries
- Standard Operation Procedures (SOP)
- Cleaning Validation
- Computer Validation
- Planning, co-ordination and execution of all qualification activities
GxP - Audit-Service
- Implementation of GxP Technical Audits
- GxP Examination of Existing Buildings and Plants
- Preparation and Support of GxP-Audits
- Quality Management Systems
- Documents
- Personnel